
RiskGONE material
Intro | Decision trees | TGs - Guidelines | Project deliverables | Data and DBs | Regulatory information | RG framework overview | SSbD
TGs - Guidelines
One of the aims of the RiskGONE project was the evaluation, adaption and validation of the existing methods for the physicochemical characterisation, human and eco-toxicological hazard assessment of ENMs. The reason for this activity is that the existing test guidelines (TGs) originally adopted for hazard and risk assessment (RA) of chemicals might not be fully applicable to nanosized materials, which present themselves with peculiar properties, or might need updates as new techniques have evolved.
RiskGONE focuses on a series of interlaboratory comparison studies (or round robins - RRs) to critically evaluate a prioritised set of existing standard operating procedures (SOPs) for their nano-specific applicability for hazard assessment. Where methodological adaptations were needed, based on detailed expert analysis and experimental testing within RiskGONE, the SOPs have been optimised and subjected to pre-validation through additional testing, whereby several RRs were performed with different panels of ENMs to confirm the repeatability and the reproducibility of the SOPs. This work aimed at generating pre-validated guidance documents (GDs) to be translated into updated or new OECD TGs.
The SOPs evaluated include methods for:
- characterization of ENMs physico-chemical parameters, such as dispersibility (ISO13318), hydrodynamic diameter and size distribution (OECD TG110), particle number, zeta potential, effective density, and endotoxin contamination (ISO 29701:2010(E)) (WP4);
- in vitro hazard identification and characterization, such as cytotoxicity (by the colony forming efficiency (CFE) assay), genotoxicity (by the micronucleus assay (OECD TG487), the mammalian cell gene mutation test (TG476), and the Comet assay (for a new in vitro TG) (WP5);
- in vivo ecotoxicity and environmental hazard assessment, such as by Daphnia magna reproduction toxicity (OECD TG211) and genotoxicity (Comet assay), as well as extension of OECD TG249 to ZF4 zebrafish embryo cells (WP6).
The consistency of the interlaboratory data and robustness of the test methods have been evaluated through statistical analyses. Nano-specific challenges (e.g., potential interference of ENMs with test methods) have been also addressed (e.g., by including interference controls to counter the issue) and, in collaboration with NanoSolveIT, they have been integrated into a decision support tool to guide users on assay suitability for specific ENM types.
Based on the obtained data, harmonised SOPs (or draft guidelines) have been prepared and made publicly available to end-users. Whenever possible and relevant, these SOPs will be translated into standard project submission forms (SPSF) to propose new OECD TGs, or amendments/annexes to existing ones. This work was aligned with other ongoing efforts such as NanoHarmony and the “Malta Initiative”, to propose revisions of ECHA’s annexes for nano-substances under REACH, and with JRC activities and other EU-funded projects (e.g., PATROLS). The SOPs and the proposed TGs will facilitate the standardisation of the hazard assessment of ENMs and will provide sustainable solutions for integrating scientific results into regulatory use beyond the life of the project.
Overview of the materials
Pre-validated guidance documents (GDs):
- D3.2. Draft guidelines regarding the quantification of lifecycle environmental and human health risk indicators
- D3.3. Draft guidelines regarding the quantification of macro-economic benefits
- D3.4. Draft guidelines regarding the correct implementation of risk transfer in insurance models
- D3.5. Draft guidelines on the societal acceptance of nanomaterials considering risk and benefit perception
- D3.6. Draft guidelines regarding an ethical impact assessment
- D3.7. Draft guidelines regarding bringing together risk indicators in an overall MCA framework
- D4.2. Consolidated pre-validated guidance document on hydrodynamic diameter and size distribution determination
- D4.3. Consolidated pre-validated guidance document on the dispersability of ENMs
- D4.4. Consolidated pre-validated guidance document on the determination of ENMs endotoxins content
- D4.5. Consolidated pre-validated guidance document on zeta potential determination
- D4.6. Consolidated pre-validated guidance document on particle counting
- D4.7. Consolidated pre-validated guidance document on effective density
- D5.1 Report on the final harmonised SOPs used to propose amendments to the existing OECD TGs, in the ANNEXES the pre-validated SOP can be found for:
- Colony Forming Efficiency CFE (also available at: Rundén-Pran et al, 2022; https://doi.org/10.3389/ftox.2022.983316)
- Comet assay (also available at El Yamani, N., Rundén-Pran, E., Collins, A.R., Longhin, E.M., Elje, E., Hoet, P., Vinković Vrček, I., Doak, S.H., Fessard, V., Dusinska, M. The miniaturized enzyme-modified comet assay for genotoxicity testing of nanomaterials. Front. Toxicol., 2022, https://doi.org/10.3389/ftox.2022.986318)
- HPRT for suspension and adherent cells
- Micronucleus assay
- D5.2 Report on the Harmonised SOPs on high throughput approaches for hazard assessment of ENMs, in the ANNEXES the pre-validated SOP can be found for:
- High content analysis of active caspase -3 and yH2AX
- Impedance-based ENM toxicity testing on adherent cells
- D6.1 Report on harmonised ecotoxicology SOPs used to propose amendments to the existing OECD TGs, in the ANNEXES the pre-validated SOP can be found for:
- Chronic (reproduction) and multigenerational toxicity to Daphnia magna
- Colony Forming Efficiency CFE highlighting adjustments for fish cell lines
- Comet assay highlighting adjustments for fish cell lines
- Impedance-based cytotoxicity assessment with fish cell lines
Additional material
Pre-validated SOP on Alamar Blue assay with NMs at: Longhin, E.M., El Yamani, M., Rundén-Pran, E., Dusinska, M. The alamar blue assay in the context of safety testing of nanomaterials. Front. Toxicol., 2022, https://doi.org/10.3389/ftox.2022.981701
RiskGONE project – effort towards standardization and pre-validation of methods for testing nanomaterials
The “technical” WPs in RiskGONE (WP4, WP5 and WP6) were focussed on developing the scientific ground to support the development of testing methods (and Test Guidelines) for ENMs. The test-method related activates undertaken in RiskGONE can be grouped into three main types:
- Standardisation and in-project validation processes, by evaluating, optimizing and pre-validating existing SOPs and TGs for their suitability for assessment of nanomaterials toxicity, and where possible we supported and expanded the efforts of the Malta project and NanoHARMONY;
- Harmonisation of methods and their reporting templates – using the same approach, and the same 6 fully-characterized ENMs dispersed utilising a common dispersion protocol, the methods developed and optimised above were evaluated in interlaboratory studies; To support the data capture, a large number of Metadata and data capture templates were developed and refined, and made available via the TemplateWizard for re-use beyond RiskGONE.
- Performance of a series of interlaboratory comparisons (2-4 round robins) to standardize and pre-validate the RiskGONE optimised methods. In each round robin at least three partners (laboratories) participated. Altogether there were 12 methods standardized and pre-validated.
At the final conference of all NMBP-13 projects (Gov4Nano, NANORIGO, RiskGONE), Gov4Nano held an international roundtable discussion towards harmonisation and standardisation of test methods. The content for this roundtable was discussed in specific standardisation worksing groups with NanoHarmony and Gov4Nano. For RiskGONE Shareen Doak (Swansea University (SU)) chaired the round table and Maria Dusinska participated in preparation of round table together with Shareen Doak and Eleonora Longhin who was rapporteur. Three projects also organised satellite Workshop on Genotoxicity also hosted by OECD – Julia Catalan (Nanorigo), Maria Dusinska and Shareen Doak (RiskGONE).
- What exactly the projects delivered regarding harmonized measurements, testing, specifications, and standards?
- RiskGONE has contributed to OECD Project 4.095 to develop a new OECD Guidance Document on adaptation of the in vitro micronucleus assay (OECD TG487) for testing of manufactured nanomaterials. This was published in Sept 2022 (Series on Testing & Assessment No. 359; ENV/CBC/MONO(2022)15).
- On OECD TG476: In Vitro Mammalian Cell Gene Mutation Tests using the Hprt and xprt genes, RiskGONE has undertaken an interlaboratory trial to determine if this TG is suitable for use with nanomaterials. The outcome of this study was that TG476 was largely robust for nanomaterials, but recommendations are needed on dispersion and characterization, maximum dose and the need for uptake analysis. These recommendations will be published in the scientific literature, which will likely happen towards the end of the RiskGONE project.
- RiskGONE also harmonized and standardized the Comet assay protocol for detection of strand breaks and oxidized DNA lesions with lesion specific enzymes, organized training for all laboratories involved and performed a series of interlaboratory studies. NILU is preparing an SPSF, and has contacted the Norwegian national coordinator and started discussion to get national support. Additional countries potentially supporting this project will be UK, Ireland, Germany, Spain, Italy.
- RiskGONE harmonized and standardized a protocol for the colony forming efficiency (CFE) cytotoxicity assay, and performed a series of interlaboratory studies. NILU is preparing an SPSF, and has contacted the Norwegian national coordinator and started discussion to get national support.
- Novel and high-throughput methods: UiB and HVL have optimized two SOPs previously established in the FP7 NANoREG project: 1) label-free impedance toxicity testing of adherent human cells in real-time, together with NILU, and 2) Toxicity testing of single human cells in suspension by impedance-based flow cytometry (UiB).
- WPMN_Adaption of OECD Test Guidelines 201, 202 and 203 for the determination of the ecotoxicity of MNs (France/Spain) LIST and UoB contributed to the activities carried out within NanoHarmony/Gov4Nano.
- Extension of OECD TG249 from Rainbow Trout gill cells to Zebrafish embryo cells and zebrafish gill/liver cells.
- Extensive work on OECD TG211 Daphnia magna reproductive (chronic) toxicity including induction of males, and potential to extend to multiple generations to assess acclimation versus increased sensitivity. Suggested adjustments for NMs include:
- Extension of the duration of the assay - NMs can delay growth & maturation & reproduction
- Comparison of freshly dispersed versus medium-aged particles
- Use of conditioned medium to account for NMs corona formation
- Addition of some extra NMs-specific analyses –
- TEM analysis of accumulation and localisation of NMs in the gut over time & damage assessment.
- ICP-MS analysis of particle loading following depuration for 24 hours (at 1, 3, 7, 14, 21 and 28 days).
- Assessment of phenotypic changes – quantification of lipid deposits, loss of tail length etc.
- Addition of AT LEAST 1 additional generation (ideally 2-3) using a paired approach – parent only exposure versus continuously exposed.
- Contribution to the Scoping review for a tiered approach for reliable bioaccumulation assessment of NMs in environmental organisms minimising use of higher tier vertebrate tests (UK).
- WNT 3.12_GD on assessing the apparent accumulation potential for NMs (Spain).
- GD317 Guidance Document on Aquatic (and Sediment) Toxicity Testing of Nanomaterials.
- Extension of WNT 4.95_GD (on the Adaptation of In Vitro Mammalian Cell Based Genotoxicity TGs for Testing of NMs (EU) – to zebrafish embryo cells - alternative models.
- New Comet assay to detect strand breaks and specific DNA lesions of Daphnia magna (or other species) exposed in vivo.
- Developed a new SOP for label-free impedance toxicity testing of adherent fish cells in real-time together with UoB and the Institute for Marine Research (IMR) in Bergen, Norway.
- Which OECD test guidelines (TG) or guidance document (GD) they launched or contributed to?
- Who developed and validated the method to became OECD TG or GD: project itself (alone) or in collaboration with e.g. Member States or support from Malta initiative?
- Project 4.095 was initiated by the JRC in 2015; in 2019 the JRC organised an expert meeting which RiskGONE partners participated in (SU & NILU). During this meeting SU (UK) agreed to take over the leadership of Project 4.095, shared with Germany (BASF/BIAC). SU & BASF/BIAC completed Project 4.095, providing the data and writing the new GD. RiskGONE was therefore instrumental in the writing of the GD, but in addition, RiskGONE has undertaken an additional interlaboratory trial to support the GD – this data was not included in the GD, but will be published as supporting and additional information.
- SU & NILU were the RiskGONE partners involved in the HPRT interlaboratory trial to determine if TG476 was appropriate for nanomaterials.
- NILU, SU, ANSES and KU Lueven performed interlaboratory studies. Standard protocol is published doi: 10.3389/ftox.2022.986318
- NILU, IMI and UiB performed interlaboratory studies (round robin). The CFE protocol is published doi: 10.3389/ftox.2022.983316
- UiB has developed the methods and initiated interlaboratory testing within the Round Robin exercises from RiskGone. Furthermore, results obtained from previous projects (NANoREG, NorNANoREG, NanoBioReal) by UiB and other laboratories (CEA in NANoREG) have been utilized to assess reproducibility and repeatability.
- In which timeframe was the method developed: when did it start when did it end?
- Project 4.095 was started in 2015 and ended in 2022.
- The interlaboratory trial for TG476 started in Sept 2019 within the RiskGONE project and the laboratory work ended in Jan 2023. The data analysis is still being finalised.
- The interlaboratory trial for the comet assay started in Sept 2019 within the RiskGONE project and the laboratory work ended in Jan 2023. The data analysis is still being finalised.
- The interlaboratory trial for CFE started in Sept 2019 within the RiskGONE project and the laboratory work ended in Jan 2023. The data analysis is still being finalised.
- The evaluation of the reliability, with regards to NM-caused interferences, of real-time label-free impedance toxicity testing on human adherent cells has started at UiB in 2011. In the FP7 project NANoREG it was tested on 23 NMs provided by JRC and project partners. It was further tested and optimized in several other projects (NFR Nano2021 NorNANoREG, NanoBioReal, EuroNanoMed II INNOCENT and GEMNS) and in RiskGONE, where an interlaboratory testing and comparison with results from previous projects was initiated. The impedance-based flow cytometry was established by UiB for nanotoxicity testing for the first time in the FP7 project NANoREG, starting from 2015. It was further optimized within RiskGONE.
- Who started the TG or GD proposal submission at Working Party of Manufactured Nanomaterials (WPMN) or Working Group of the National Coordinators for the Test Guidelines Programme (WNT) in OECD?
- JRC initiated Project 4.095 to write the new GD, but in 2019 SU (UK) took over the leadership via RiskGONE with Germany, to complete the project.
- Gene mutation test did not lead to modification of the TG as only concentration range and exposure time need to be adjusted. Additionally need for uptake should be added potentially; this could be suggested in the future for a slight modification of the TG476 or be positioned within a separate nano-genotoxicity testing Guidance Document.
- Comet assay is under preparation for SPSF to be submitted in November 2023. NILU is responsible
- CFE is under preparation for SPSF to be submitted in November 2023. NILU is responsible
- The national contact point was contacted, UiB is currently planning the roadmap towards submission
- Who has done the (pre)validation of the method in view that OECD accept only validated methods?
- SU and Germany did the initial prevalidation that was included in the GD (SU did this under the RiskGONE project); in addition a further interlaboratory trial has been conducted through RiskGONE (including partners SU, NILU & ANSES), which will be published to add additional experimental evidence to support the new GD.
- SU & NILU were the RiskGONE partners involved in the HPRT interlaboratory trial to determine if TG476 was appropriate for nanomaterials.
- NILU, SU, ANSES and KU Lueven performed interlaboratory studies. Standard protocol is published doi: 10.3389/ftox.2022.986318
- NILU, IMI and UiB performed interlaboratory studies (round robin). The CFE protocol is published doi: 10.3389/ftox.2022.983316
- UiB is in charge for the novel and high-throughput methods
- What were the project steps from development to validation?
- RiskGONE came into the Project 4.095 at the stage of establishing a harmonised protocol, that was subsequently trailed through interlaboratory testing.
- For TG476 interlaboratory trial, within RiskGONE first a harmonised protocol was established that would be applied in the round robin testing. A set of appropriate materials were identified to trial in the first round robin. Once round robin-1 had been completed, we analysed the data across the laboratories involved in the trial and established that the method was robust for the small number of materials evaluated. Round Robin-2 was these designed to expand the range of nanomaterials tested by the protocol.
- For Comet assay interlaboratory trial, within RiskGONE first a harmonised protocol was established that would be applied in the round robin testing. A set of appropriate materials were identified to trial in the first round robin. Once round robin-1 had been completed, we analysed the data across the laboratories involved in the trial and established that the method was robust for the small number of materials evaluated. Round Robin-2 was these designed to expand the range of nanomaterials tested by the protocol
- For CFE interlaboratory trial, within RiskGONE first a harmonised protocol was established that would be applied in the round robin testing. A set of appropriate materials were identified to trial in the first round robin. Once round robin-1 had been completed, we analysed the data across the laboratories involved in the trial and established that the method was robust for the small number of materials evaluated. Round Robin-2 was these designed to expand the range of nanomaterials tested by the protocol
- A comparison of methods and round robin testing has been performed in WP5 (Tasks 5.2 and 5.1). Furthermore, impedance-based testing has been done on both human and fish cells, the latter together with UoB and IMR to evaluate its suitability for different cell types, as well as for human and ecotoxicology.
- What was already done before the project started and what are the planned activities – if any - after the project duration?
- Prior to RiskGONE, the JRC had produced standardised nanomaterials that were to be used in the interlaboratory trial. They had also characterised the physico-chemical features of these materials and established if the cells to be used in the interlab trial were able to internalise them. JRC organised the expert meeting in which the harmonised protocol was defined and at which stage SU & Germany took the project over. RiskGONE (via SU and together with Germany) then completed project 4.095, producing the OECD GD. The only activity planned that may run beyond the project duration is generating a publication of the interlaboratory trial that was conducted by RiskGONE partners in support of the new GD.
- Prior to RiskGONE, no work had been initiated to explore the validity of TG476 for nanomaterials. This was done entirely within RiskGONE. The outcome of this study was that TG476 was largely robust for nanomaterials, but recommendations are needed on dispersion and characterization, maximum dose and the need for uptake analysis. These recommendations will be published in the scientific literature, which will likely happen towards the end of the RiskGONE project.
- Several efforts have been done for the comet assay and pre validation studies – ESCODD, COMICS project, hComet COST action and other projects for standard chemicals. Within NanoREG, NanoReg2, NorNanoREG and other projects comet assay was further standardised to test nanomaterial. First pre-validation and interlaboratory studies are performed on nanomaterials within RiskGONE,
- CFE was pre-validated by JRC and further pre-validated within RiskGONE.
- The methods have been used and further developed and optimized within several projects (FP7 NANoREG, NFR Nano2021 NorNANoREG, NanoBioReal, EuroNanoMed II INNOCENT and GEMNS). Currently, they are used in an EEA project (TEPCAN) and an INRAE project (NanoMilk) and will be used in future projects.
RiskGONE has developed methods for characterisation, transformation, fate, and dosimetry, including dispersibility of ENMs, determination of surface charge of ENMs, determination of endotoxin content of ENMs, determination of ENM particle number in solution, determination of hydrodynamic diameter and size distribution of ENMs in water and in biological media, developed a protocol for re-suspension of ENMs in water and in biological media, and performed harmonized measurements and participated in round robins (within WP4). The ENMs tested and the methods developed, optimised and Round-Robin tested are summarised in the figure below.

With regard to human hazard (WP5):
For environmental hazard (WP6) the following endpoints and methods have been addressed:
RiskGONE contribution to work towards OECDD WPMN/WNT
| TG/GD | RiskGONE contribution (partner mostly involved) |
|---|---|
| WNT 4.133_Applicability of the key event based TG 442D for in vitro skin sensitisation testing of NMs (Switzerland) | Adaptation for ENMs. LIST is supporting its activities through the work carried out within Gov4Nano. LIST took already contact with TEMASOL and BAG (Sabine Frey) |
| WNT 1.4_TG on particle size and size distribution of MNs (GER) | Guidance document for size distribution, under the MALTA initiative (WP4 partners). We performed round robins on ENMs dispersed in biological media and this part could be added to the GD under preparation. In addition, we are working on the definition of a SOP for dispersion of samples based on the methods by Deloid et al. Nat Protoc. 2017 Feb;12(2):355-371. doi: 10.1038/nprot.2016.172. Epub 2017 Jan 19. |
| Solubility/Dissolution | |
| TG201 | Adaptation of TG to ENMs, lead by Spain, in collaboration with NanoHarmony (LIST, UoB) |
| TG202 | Adaptation of TG to ENMs, lead by Spain, in collaboration with NanoHarmony (LIST, UoB) |
| TG203 | Adaptation of TG to ENMs, lead by Spain, in collaboration with NanoHarmony (UoB) |
| OECD Project 4.95, Adaptation of the in vitro micronucleus assay (OECD TG487) led by JRC | Adaptation for testing of manufactured nanomaterials. Published in Sept 2022 (Series on Testing & Assessment No. 359; ENV/CBC/MONO(2022)15) (SU) |
| TG211 | Adaptation for testing of manufactured nanomaterials |
| TG249 | Adaptation for testing of manufactured nanomaterials: extension of the assay |
| WNT 3.12_GD on assessing the apparent accumulation potential for NMs (Spain) | Contribution with data |
| GD317 Guidance Document on Aquatic (and Sediment) Toxicity | Adaptation for the testing of nanomaterials |
Additional contribution that is not yet part of the OECD WPMN/WNT
| TG/GD | RiskGONE contribution (partner mostly involved) |
|---|---|
| OECD TG476: In Vitro Mammalian Cell Gene Mutation Tests using the Hprt and xprt genes | RiskGONE established that TG476 was largely robust for ENMs, but recommendations are needed on dispersion and characterization, maximum dose and the need for uptake analysis. These recommendations will be published in the scientific literature, which will likely happen towards the end of the RiskGONE project (SU, NILU) |
| No TG/GD available, In vitro Comet assay with lesion specific enzymes | Interlaboratory studies with ENMs performed, SOPs was adapted for ENMs built on protocol standardized within FP7 NanoTEST and NanoREG, , protocol published, Template for data collection developed, SPSF under preparation |
| No TG/GD available, CFE (CFE, was pre-validated by JRC) | Interlaboratory studies with ENMs performed, SOPs was adapted for ENMs built on JRC protocol and NILU miniaturization, protocol published, Template for data collection developed, SPSF under preparation |
| No TG/GD available, label-free impedance toxicity testing of adherent human cells in real-time | Studies with ENMs, protocol standardized |
| No TG/GD available, single human cells in suspension by impedance-based flow cytometry | Studies with ENMs, protocol standardized |
| No TG/GD available, new Comet assay to detect strand breaks and specific DNA lesions of Daphnia magna (or other species) exposed in vivo | Studies with ENMs, protocol standardized, Template for data collection developed, SPSF under preparation |
| Daphnia magna reproduction test (with male induction) | Protocol standardised, Template for data collection developed, SPSF under preparation |
| Scoping review for a tiered approach for reliable bioaccumulation assessment of NMs in environmental organisms minimising use of higher tier vertebrate tests (UK) | UoB already took contact with Up and is already feeding into this, Template for data collection developed, SPSF under preparation |
| No TG/GD available, determination of Endotoxin content | Protocol standardized, interlaboratory studies performed, SOPs was adapted for ENMs built on ISO and NanoREG, Template for data collection developed |
| Possibly addition/amendment to WNT 1.4_TG on particle size and size distribution of MNs lead by Germany | Technical recommendation to perform size and size distribution of MNs in biological media. Interlaboratory studies with ENMs performed, Template for data collection developed |
| Possibly addition/amendment to WNT 1.4_TG on particle size and size distribution of MNs lead by Germany | Technical recommendation on how to perform particles counting with NTA. Interlaboratory studies with ENMs performed, Template for data collection developed |
| Possibly addition/amendment to WNT 1.4_TG on particle size and size distribution of MNs lead by Germany | Zeta-potential. Interlaboratory studies with ENMs performed, Template for data collection developed |
For ENMs characterization: all activities linked to supporting TG/GD, they were all linked to OECD projects, some member state was involved. Certain activities were also supported by the MALTA initiative, but when you are at WNT or WPMN, then at least one member state (or the OECD secretariat or the EC) must be a sponsor of the project.
For human hazard:
For environmental hazard: Several SOPs were in collaboration with Malta Initiative and NANOHARMONY, and several activities started within RiskGONE. As the WP6 did not have enough partners, for round robins external partners have been found.
Most of the projects we supported started well before RiskGONE, but accelerated in the RiskGONE as we were conducting interlaboratory studies. There is one finished method and it is the GD on size distribution. All others are ongoing or barely started (e.g. the eco-tox ones barely started the ILCs)
For human hazard:
For environmental hazard: Within RiskGONE, an SOP was developed together with UoB and the Institute for Marine Research in Bergen for fish adherent cells. This work started in 2020. Some methods started before the RiskGONE but several such as comet assay for Daphnia started within RiskGONE.
RiskGONE contributed to ongoing activities within Malta initiative and NANOHARMONY - for those which were already submitted. Individual partners of RiskGONE such as LIST, University of Birmingham or Swansea University participated as supporting or leading countries for specific SPSF. List of approve SPSF or TGs/DGs can be found on WPMN website.
For ENMs characterization: the development of the GD on size distribution was initiated by Germany, in collaboration with the MALTA initiative and the Gov4Nano project.
For human hazard:
For environmental hazard: Adaptation of the TG201, TG202 and TG203 was initiated by Spain with the support of the MALTA initiative and of the NanoHarmony H2020 project.
The preparatory work – interlaboratory studies were carried out within RiskGONE. At least 12 methods have been prevalidated by RiskGONE – 6 for WP4 (Characterisation). Some methods are being prepared for SPSF, some were already accepted as OECD projects and we joined these activities by providing data from our round robins.
For human hazard:
For environmental hazard: The preparatory work – interlaboratory studies were carried out within RiskGONE. Several methods have been pre-validated by RiskGONE related to environmental hazard. Some methods are being prepared for SPSF, some were already accepted as OECD projects and we joined these activities by providing data from our round robins.
Before submission of SPSF within RiskGONE we selected SOPs, harmonised and standardised, did training where needed and run interlaboratory studies. Other steps then are: SPSF submission, approval by WNT/WPMN, establishment of expert group, definition of SOP, organization and running of ILCs, data analysis, reporting.
For human hazard:
For environmental hazard: Before submission of SPSF within RiskGONE we selected SOPs, harmonised and standardised, did training where needed and run interlaboratory studies. Other steps then are: SPSF submission, approval by WNT/WPMN, establishment of expert group, definition of SOP, organization and running of round robins, data analysis, reporting.
For those methods that are developed inside RiskGONE, we started to work within the project itself. Some methods were developed in previous projects and we developed them further within RiskGONE.
Specifically for human hazard:
Additionally – interlaboratory trials is ongoing for advanced in vitro models – lung (air liquid interface ALI) and liver (liver spheroids).
For environmental hazard: For those methods that are developed inside RiskGONE, we started to work within the project itself. Some methods were developed in previous projects and we developed them further within RiskGONE.
RiskGONE deliverables related to standardisation
WP4 deliverables on ENM characterisation
- D4.1. Report on the rounds of the RRs for characterisation of ENMs. (M22, M28, M34) (LIST)
- D4.2. Consolidated pre-validated guidance document on hydrodynamic diameter and size distribution determination. (M36) (CID)
- D4.3. Consolidated pre-validated guidance document on the dispersibility of ENMs. (M36) (CSIC)
- D4.4. Consolidated pre-validated guidance document on the determination of ENMs endotoxins content. (M36) (NILU)
- D4.5. Consolidated pre-validated guidance document on zeta potential determination. (M36) (CSIC)
- D4.6. Consolidated pre-validated guidance document on particle counting. (M36) (LIST)
- D4.7. Consolidated pre-validated guidance document on effective density. (M36) (CID)
- D4.8. Report on the applicability of OECD TGs for determination of the environmental fate of ENMs. (M42) (CID)
- D4.9. Harmonised SOP for the resuspension of ENMs in biological media and in vitro dosimetry. (M30) (IMI)
- D4.10. Report on the training material distributed to WP2 and to WP7 (M22 – M32 – M54) (CSIC)
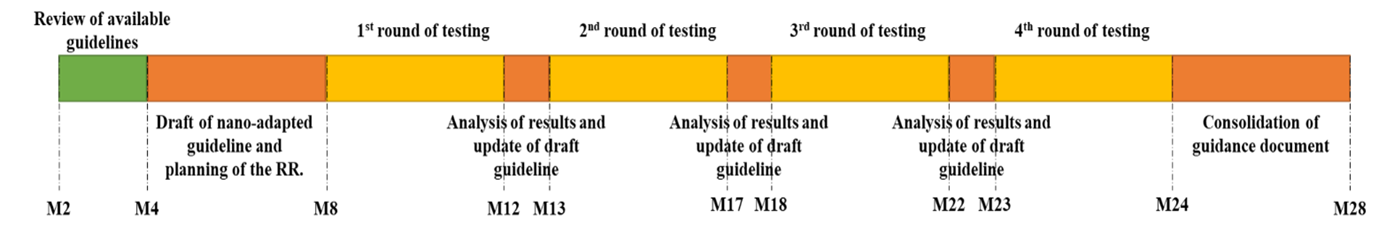
WP5 deliverables on human hazard assessment
- D5.1. Report on the final harmonised SOPs used to propose amendments to the existing OECD TGs. (M36) (NILU)
- D5.2. Report on the harmonised SOPs on high throughput approaches for hazard assessment of ENMs. (M48) (UiB)
- D5.3. Report on nano-specific sex differences to direct future hazard assessment approaches. (M42) (IMI)
- D5.4. Report on the proof of concept evaluation of SOPs for innovative in vitro models and mechanistically relevant assays for nanosafety human hazard assessment. (M46) (SU)
- D5.5. Report on the expert meeting AOP draft review. (M22 and M42) (KU Leuven)
- D5.6. End-user training materials for users and the research community. (M30 and M46) (SU)
WP6 deliverables on ecotoxicological hazard assessment
- D6.1. Report on the final harmonised SOPs used to propose amendments to the existing OECD TGs. (M36) (UoB)
- D6.2. Report on pre-validated high throughput (in vitro) and miniaturised (in vivo) methods for ecotoxicity testing. (M48) (HVL)
- D6.3. Documented protocols, data capture, and meta data templates for revised OECD tests, and pre-validated alternative test methods. (M42) (LIST)
- D6.4. Report on feedback on an initial draft of DEB-based AOP for chronic ecotoxicity to ENMs from a workshop at M24. (M26) (UoB)
- D6.5. Report on DEB-based AOP for chronic ecotoxicity to ENMs, and extension to multi-generational effects. (M42) (UoB)
- D6.6. Report on nano-specific gender differences to direct future ERA approaches. (M42) (IMI)
- D6.7. End-user training materials for users and research community. (M30 and M46) (QSARL).